Do you want to find 'write a balanced equation for the combustion of methanol'? You can find questions and answers on the topic here.
The balanced chemical equivalence for the burning of liquid wood alcohol in oxygen accelerator pedal to yield C dioxide gas and liquid water is: 2CH3OH(l) + 3O2(g) → 2CO2(g) + 4H2O(l)
Table of contents
- Write a balanced equation for the combustion of methanol in 2021
- Burning methanol
- Methanol equation
- Use bond energies to calculate the enthalpy of combustion of methanol in kj/mol.
- Ch3oh+o2=co2+h2o
- Combustion of ch3oh
- Ch3oh balanced equation
- Incomplete combustion of methanol
Write a balanced equation for the combustion of methanol in 2021
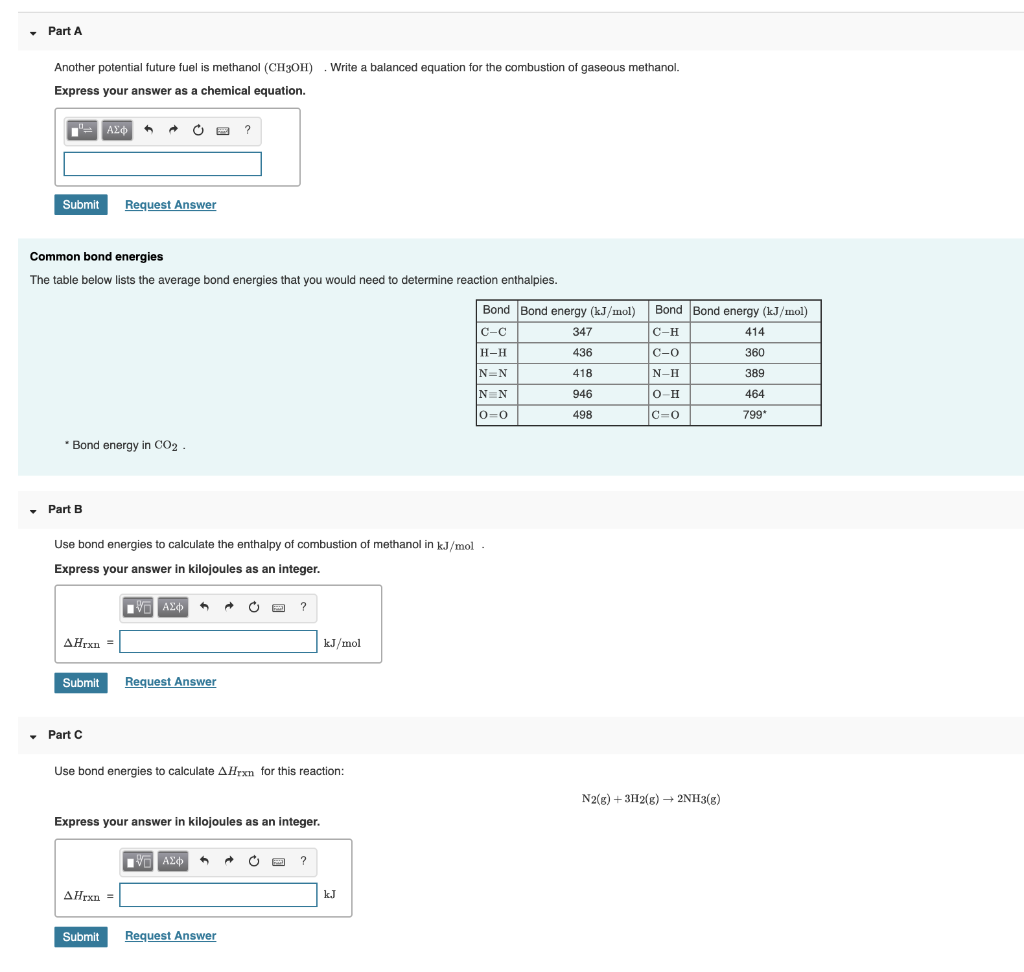 This image representes write a balanced equation for the combustion of methanol.
This image representes write a balanced equation for the combustion of methanol.
Burning methanol
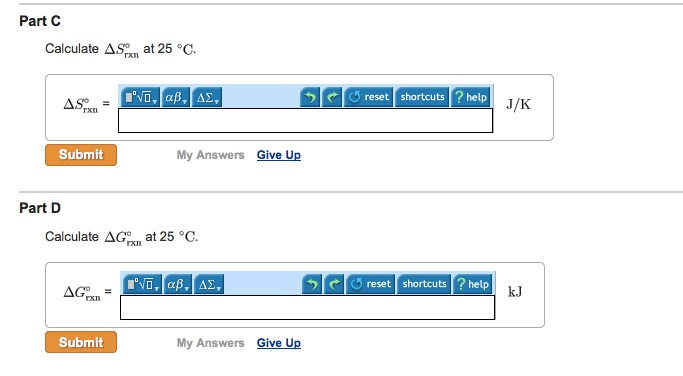 This image representes Burning methanol.
This image representes Burning methanol.
Methanol equation
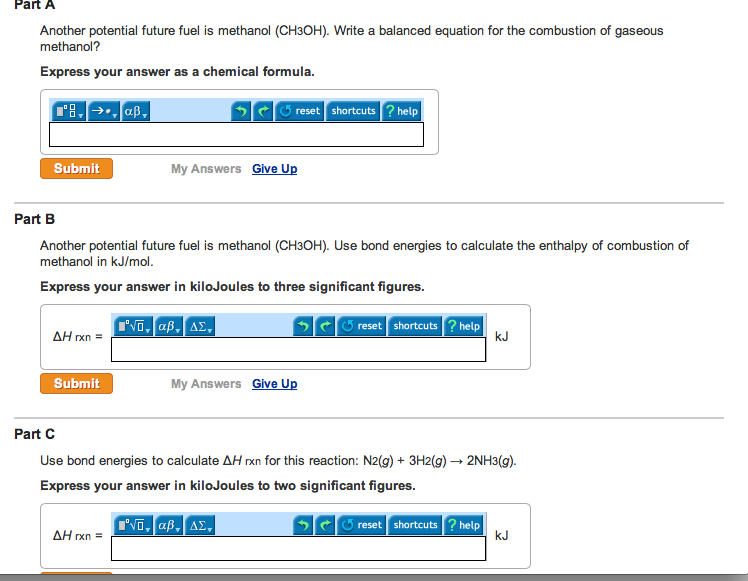 This picture illustrates Methanol equation.
This picture illustrates Methanol equation.
Use bond energies to calculate the enthalpy of combustion of methanol in kj/mol.
 This image representes Use bond energies to calculate the enthalpy of combustion of methanol in kj/mol..
This image representes Use bond energies to calculate the enthalpy of combustion of methanol in kj/mol..
Ch3oh+o2=co2+h2o
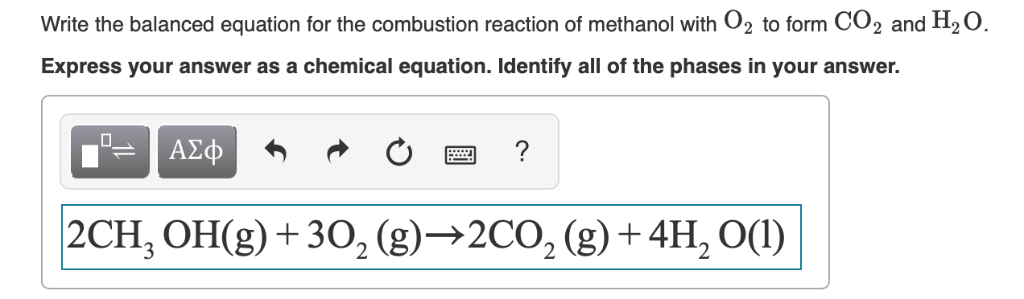 This image demonstrates Ch3oh+o2=co2+h2o.
This image demonstrates Ch3oh+o2=co2+h2o.
Combustion of ch3oh
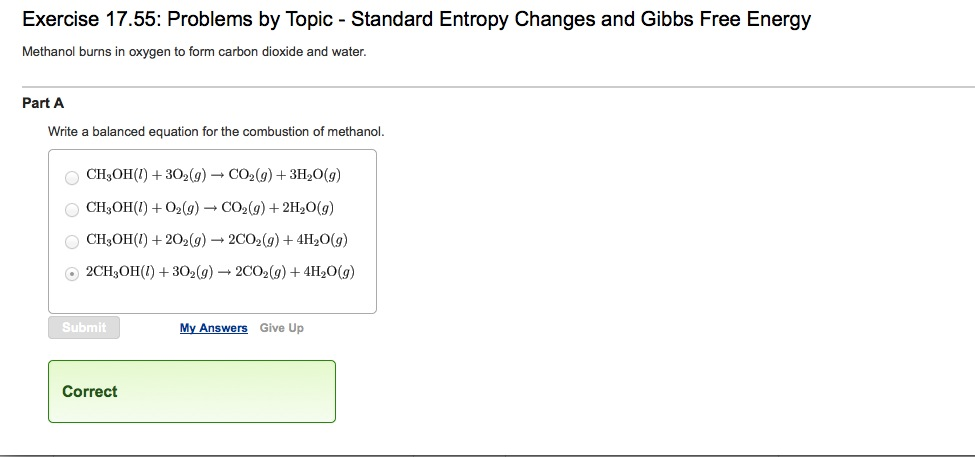 This picture demonstrates Combustion of ch3oh.
This picture demonstrates Combustion of ch3oh.
Ch3oh balanced equation
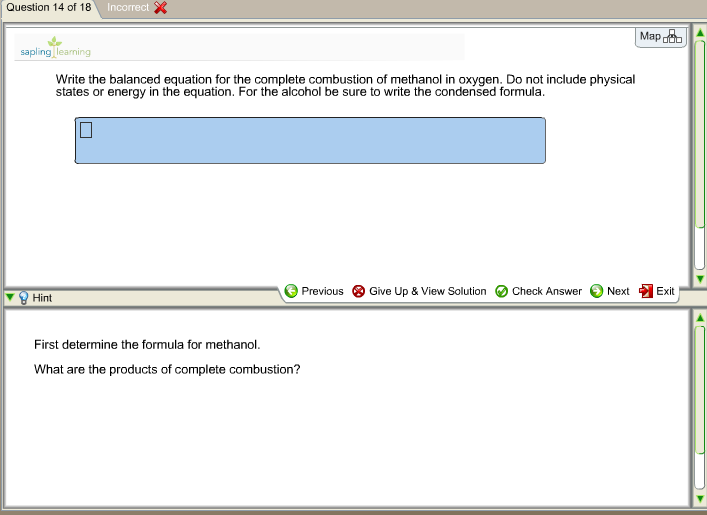 This image demonstrates Ch3oh balanced equation.
This image demonstrates Ch3oh balanced equation.
Incomplete combustion of methanol
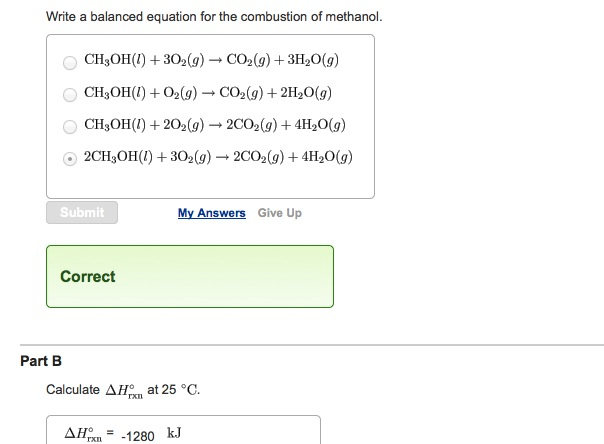 This image demonstrates Incomplete combustion of methanol.
This image demonstrates Incomplete combustion of methanol.
How to write out balanced reactions for alcohol?
Odd-numbered alcohols require non-integral stoichiometric coefficients.....you can always remove the 1 2 coefficient by doubling the equation. n = 1,2,3... is the number of carbon atoms in the alcohol.
Which is the correct equation for balancing O2 and X?
The n + 1 makes for a difficult balancing act, so let's double everything to see a little better, and multiply O2 by x to balance using that next, x ≠ n. Now we have 6n + 2 oxygen atoms on the right-hand side, and 2 +4x on the left-hand side. Thus, we construct another quick balancing equation: Thus, our equation is balanced in general.
What is the equation for the combustion of methanol?
Methanol’s chemical formula is CH3OH so the basic equation to burn this fuel in oxygen would be: nCH3OH + X O2 = n CO2 + 2n H2O. where n is the number of moles of methanol and X is the number of moles of oxygen required for complete combustion or methanol.
What is the balanced equation for the complete combustion?
What is the balanced equation for the complete combustion of methanol? ... Q: What is the balanced equation for the complete combustion of methanol? Write your answer...
Last Update: Oct 2021