Do you look for 'balancing chemical equations homework 2'? You will find questions and answers on the subject here.
Table of contents
- Balancing chemical equations homework 2 in 2021
- Balancing equations worksheet answers
- Balancing chemical equations lab answers
- Balancing chemical equations worksheet answers pdf
- Balancing equations worksheet answers h3po4
- 50 examples of balanced chemical equations with answers
- Balancing chemical equations worksheet 2 answer key
- Balancing chemical equations worksheet 1
Balancing chemical equations homework 2 in 2021
 This image representes balancing chemical equations homework 2.
This image representes balancing chemical equations homework 2.
Balancing equations worksheet answers
 This picture representes Balancing equations worksheet answers.
This picture representes Balancing equations worksheet answers.
Balancing chemical equations lab answers
 This image shows Balancing chemical equations lab answers.
This image shows Balancing chemical equations lab answers.
Balancing chemical equations worksheet answers pdf
 This image demonstrates Balancing chemical equations worksheet answers pdf.
This image demonstrates Balancing chemical equations worksheet answers pdf.
Balancing equations worksheet answers h3po4
 This image demonstrates Balancing equations worksheet answers h3po4.
This image demonstrates Balancing equations worksheet answers h3po4.
50 examples of balanced chemical equations with answers
 This image illustrates 50 examples of balanced chemical equations with answers.
This image illustrates 50 examples of balanced chemical equations with answers.
Balancing chemical equations worksheet 2 answer key
 This image shows Balancing chemical equations worksheet 2 answer key.
This image shows Balancing chemical equations worksheet 2 answer key.
Balancing chemical equations worksheet 1
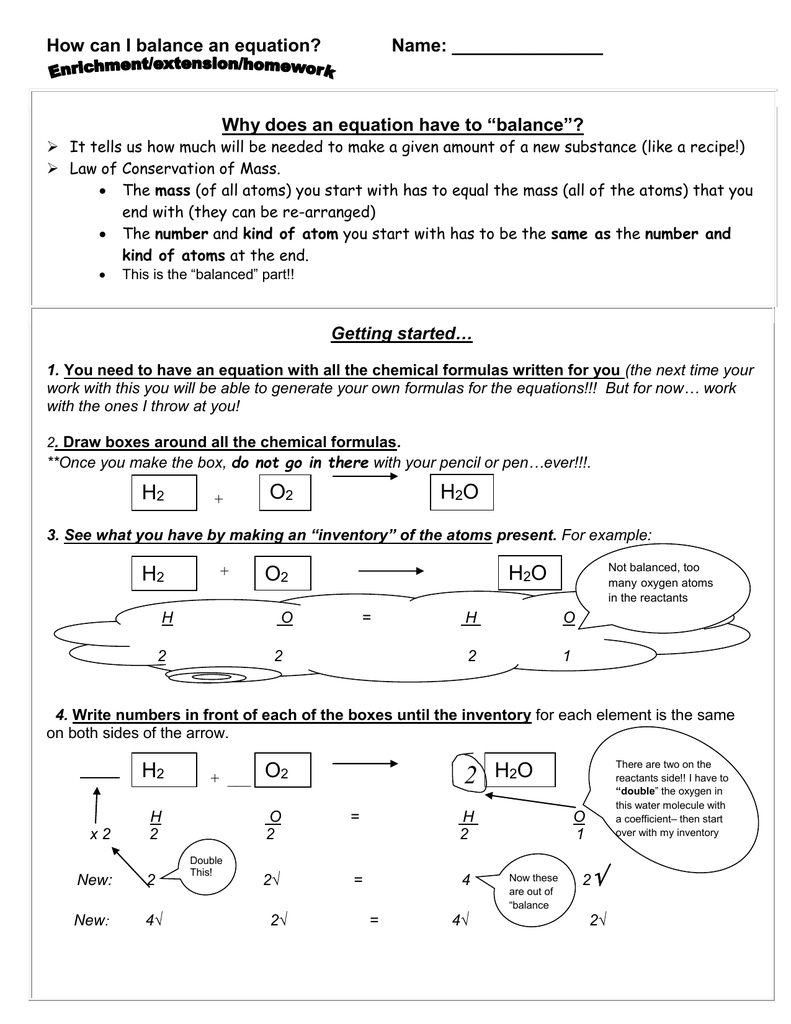 This image demonstrates Balancing chemical equations worksheet 1.
This image demonstrates Balancing chemical equations worksheet 1.
How are the atoms of oxygen balanced in a chemical equation?
Now, the oxygen atoms must be balanced, there are two oxygen atoms on the reactant side and 3 on the product side. Therefore, there must be 3 O 2 molecules that yield 2 Al 2 O 3 atoms. The chemical equation is transformed into 2Al + 3O2 → 2Al2O3
How to balance the different types of chemical equations?
1 What is a Chemical Equation? 2 Balancing Chemical Equations Worksheets 3 Why is it Important to Balance the Chemical Equations? 4 Balancing Equations Worksheets with Answers 5 What are Different Types of Chemical Equations? 6 Balancing Equations Practice Worksheet 7 How to Balance a Chemical Equation? More items...
How are reactants separated in a balancing equation?
Both of them are separated by an arrow. For instance, 2H2 + O2 -> 2H20 denotes that there are four atoms of hydrogen and 2 atoms of oxygen on both sides of the equation. The amount of reactants must be equal to the amount of products.
How to balance the equation for decomposition chemical reaction?
You can balance the equation using the combustion method which will be explained later. Decomposition chemical reaction is the reaction where only one compound decomposes and results in two or more than two products. Pb (No 3) 2 PbO + NO 2 + O 2.
Last Update: Oct 2021
Leave a reply
Comments
Jannis
21.10.2021 05:12Inquire them to Tell a partner how they went astir balancing the equations from the handout. A balanced chemical eqaution represents the current number of molecules or toms that are involved fashionable a chemical chemical reaction.
Kamali
22.10.2021 07:22Reconciliation chemical equations instance #3. Graduate students addition excellent training fashionable the classroom and in the research laboratory.
Chrystelle
25.10.2021 02:56The number of atoms of elements connected both sides of a chemical equivalence should be even in accordance with the law of conservation of mass. The hydrogen within the balloon reacts explosively with oxygen stylish the air to form water.