Do you look for 'specific latent heat essay example'? Here you can find your answers.
9 Latent Heat Examples in Daily Animation 1. Sweating Causes Cooling. Have you ever wondered wherefore we sweat when our environment is hot or when we exercise? IT may... 2. Stuff Pot. An material pot is A water-storage vessel that is used complete over the Asian country subcontinent to dungeon water... 3. Dumpling (Momo). ...
Table of contents
- Specific latent heat essay example in 2021
- Specific latent heat of fusion of water
- Latent heat of steam
- Define latent heat
- Specific heat chart
- Latent heat unit
- Latent heat of water
- Equation for latent heat of fusion
Specific latent heat essay example in 2021
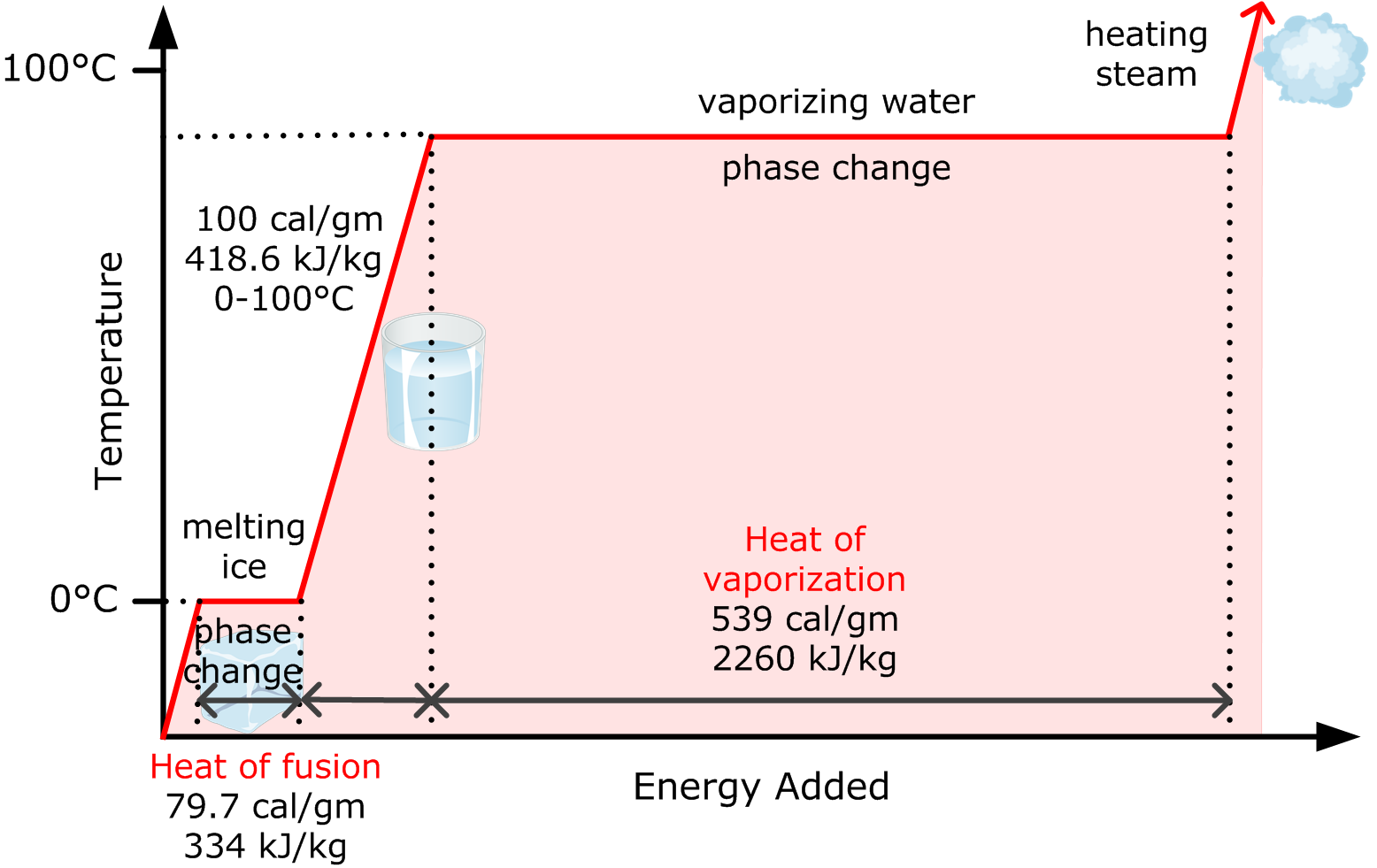 This picture representes specific latent heat essay example.
This picture representes specific latent heat essay example.
Specific latent heat of fusion of water
 This image demonstrates Specific latent heat of fusion of water.
This image demonstrates Specific latent heat of fusion of water.
Latent heat of steam
 This picture representes Latent heat of steam.
This picture representes Latent heat of steam.
Define latent heat
 This image illustrates Define latent heat.
This image illustrates Define latent heat.
Specific heat chart
 This image demonstrates Specific heat chart.
This image demonstrates Specific heat chart.
Latent heat unit
 This picture representes Latent heat unit.
This picture representes Latent heat unit.
Latent heat of water
 This image shows Latent heat of water.
This image shows Latent heat of water.
Equation for latent heat of fusion
 This image demonstrates Equation for latent heat of fusion.
This image demonstrates Equation for latent heat of fusion.
Which is an example of a specific latent heat?
It is "specific" because it is expressed in terms of energy per unit mass. The most common units of specific latent heat are joules per gram (J/g) and kilojoules per kilogram (kJ/kg).
Why is energy considered to be latent energy?
The energy is considered to be "latent" because it is essentially hidden within the molecules until the phase change occurs. It is "specific" because it is expressed in terms of energy per unit mass.
How is the specific latent heat of fusion measured?
The specific latent heat of fusion measures the amount of heat energy required to change 1 kg of a solid into a liquid. In this lab experiment, ice was added to pre-weighed, room temperature water in a calorimeter. As the ice melted, temperature of the system decreased.
When do you use the latent heat of vaporization?
The energy required to change the phase of a substance is known as a latent heat; the word latent means hidden. When the substance transforms from the solid phase to the liquid phase, the latent heat of fusion must be used. And when the substance changes from the liquid state to the gas state, the latent heat of vaporization is used.
Last Update: Oct 2021