Are you ready to find 'how to write an electron configuration'? Here you can find all of the details.
Table of contents
- How to write an electron configuration in 2021
- Electron configuration of potassium
- Electronic configuration of elements pdf
- Writing electron configurations chart
- How to electron configuration notation
- Electronic configuration of all elements
- Electronic configuration of sodium
- Electron configuration of carbon
How to write an electron configuration in 2021
 This picture illustrates how to write an electron configuration.
This picture illustrates how to write an electron configuration.
Electron configuration of potassium
 This image representes Electron configuration of potassium.
This image representes Electron configuration of potassium.
Electronic configuration of elements pdf
 This image shows Electronic configuration of elements pdf.
This image shows Electronic configuration of elements pdf.
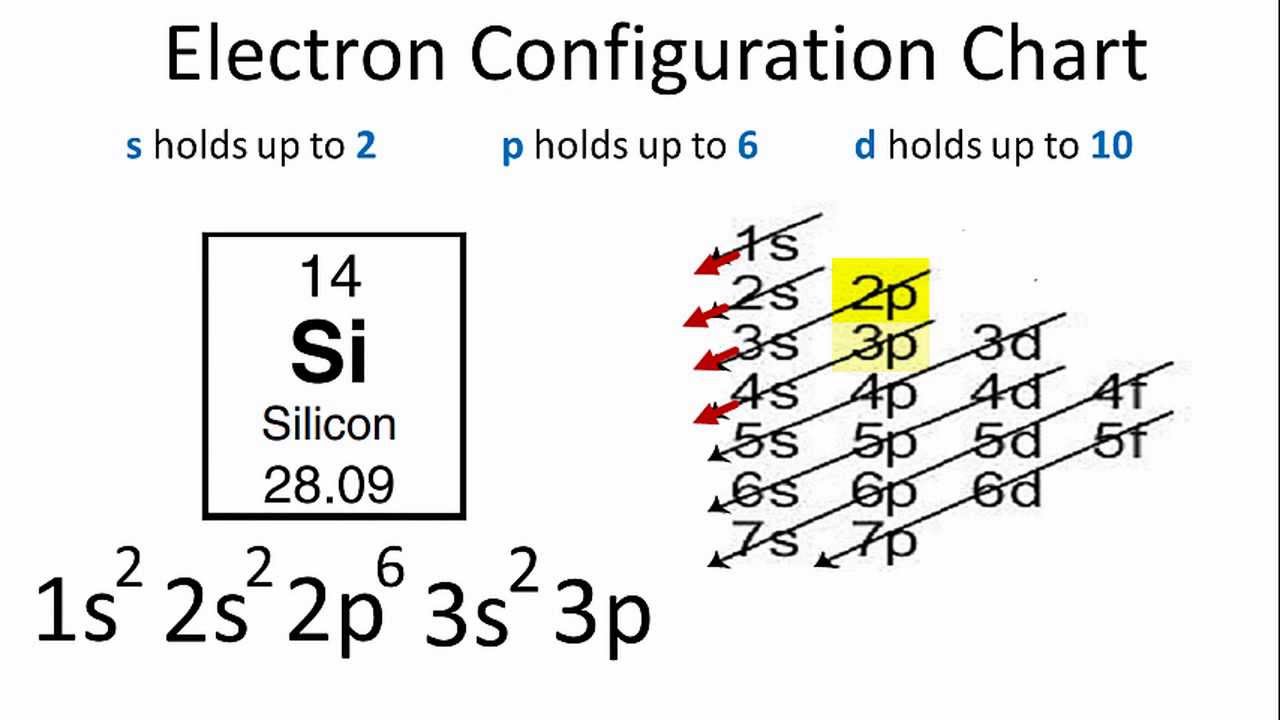
Writing electron configurations chart
 This picture representes Writing electron configurations chart.
This picture representes Writing electron configurations chart.
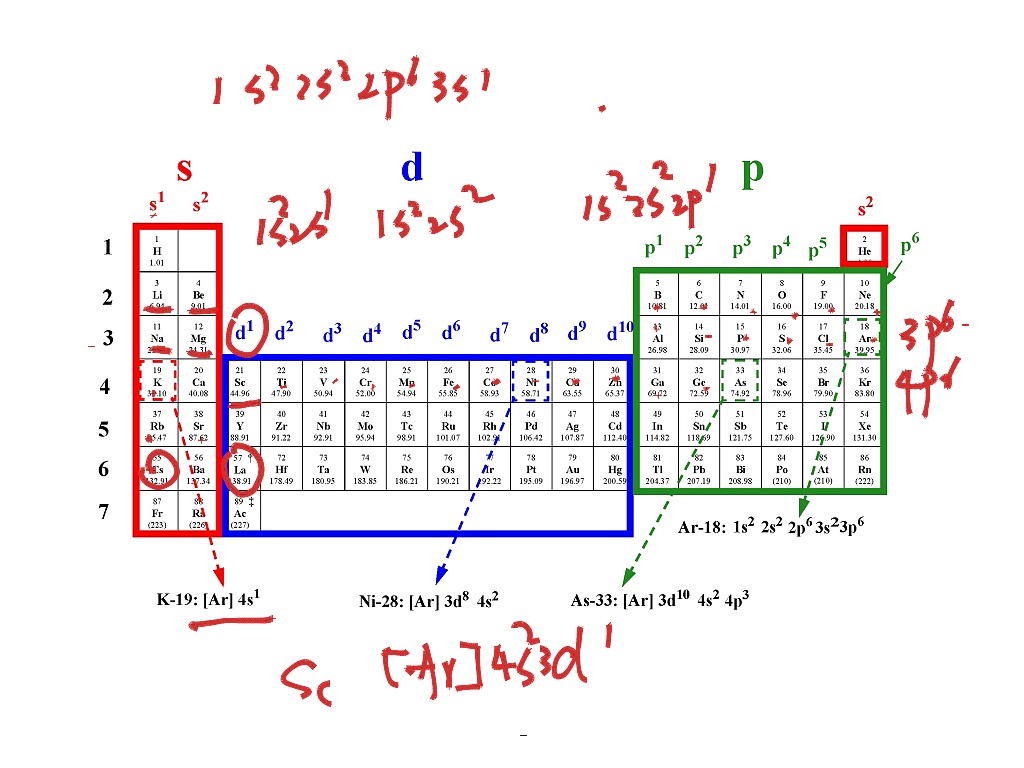
How to electron configuration notation
 This picture representes How to electron configuration notation.
This picture representes How to electron configuration notation.
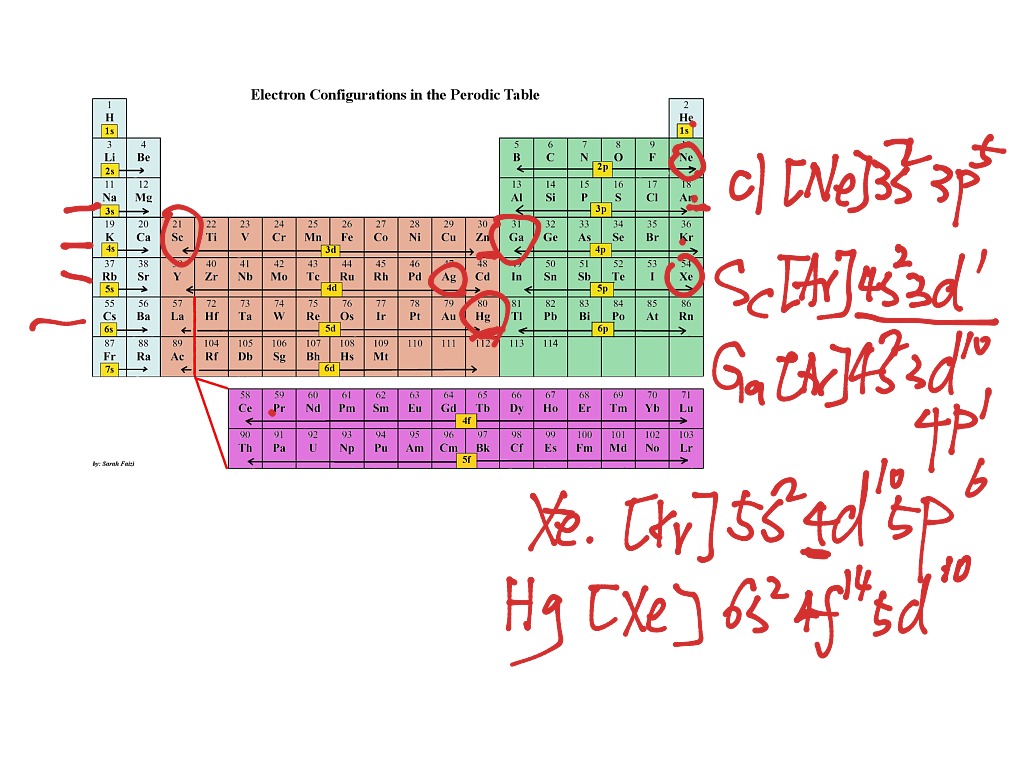
Electronic configuration of all elements
 This picture demonstrates Electronic configuration of all elements.
This picture demonstrates Electronic configuration of all elements.
Electronic configuration of sodium
 This picture demonstrates Electronic configuration of sodium.
This picture demonstrates Electronic configuration of sodium.
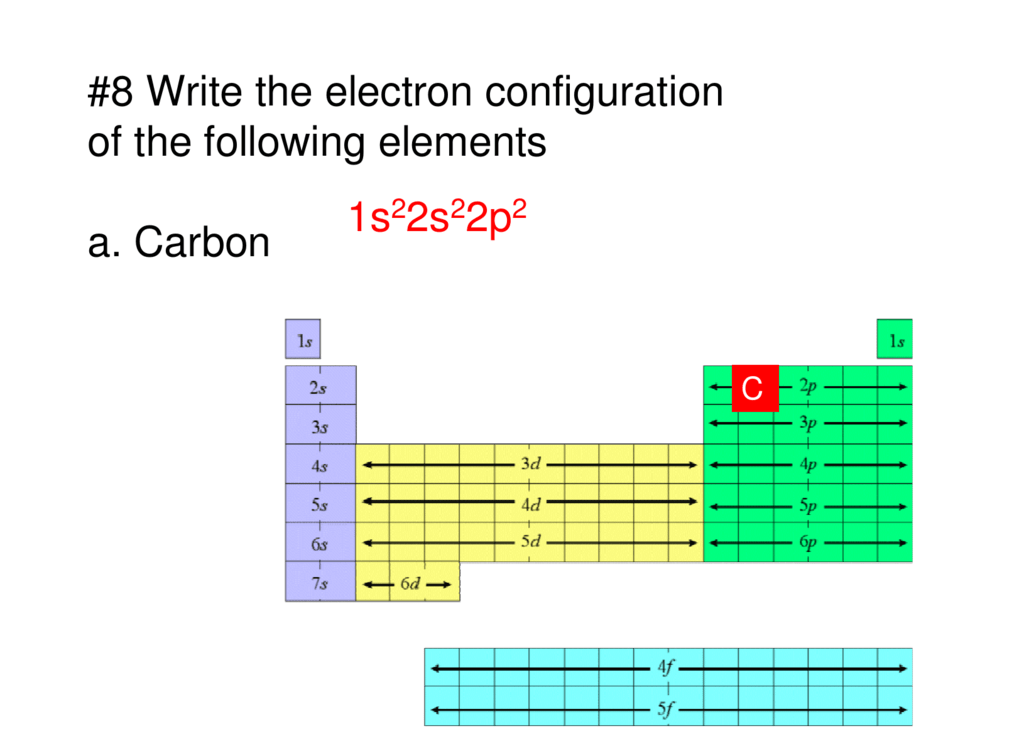
Electron configuration of carbon
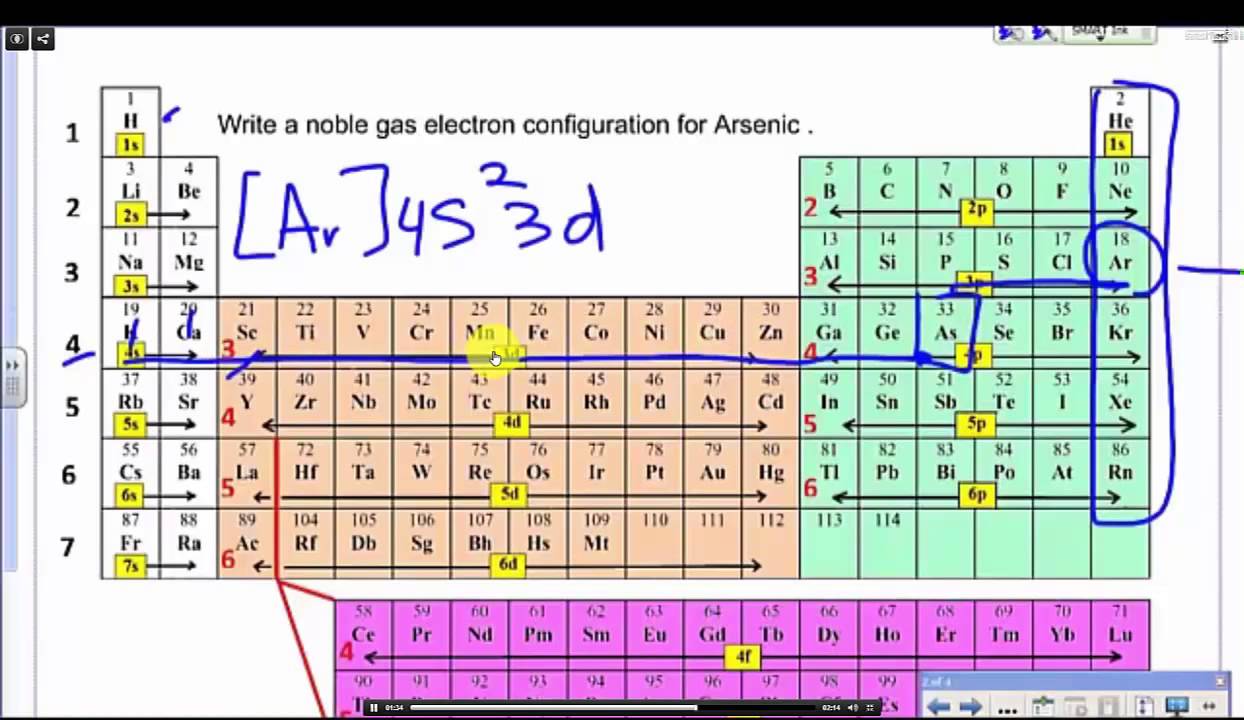 This picture representes Electron configuration of carbon.
This picture representes Electron configuration of carbon.
How is the electronic configuration of an element written?
The electronic configuration of an element is a symbolic notation of the manner in which the electrons of its atoms are distributed over different atomic orbitals. While writing electron configurations, a standardized notation is followed in which the energy level and the type of orbital are written first, followed by the number of electrons ...
How is the configuration of an atom defined?
Electronic configuration is defined as the distribution of electrons into the orbitals of an atom. Every neutral atom consists of a fixed number of electrons which is equal to the number of protons and is called the atomic number.
How do you write an electron shell configuration?
Remember, for electron configurations you work left to right and down the periods until you get to the element you’re focusing on. The last noble gas that was passed for bromine was argon (Ar). Using the short-hand method, we will place argon in brackets like this [Ar] and then continue the electron configuration after argon.
How is an electron configuration written in superscript?
While writing electron configurations, a standardized notation is followed in which the energy level and the type of orbital are written first, followed by the number of electrons present in the orbital written in superscript. For example, the electronic configuration of carbon (atomic number: 6) is 1s22s22p2.
Last Update: Oct 2021
Leave a reply
Comments
Latangie
28.10.2021 10:26The essay rubric for the project evaluation. How to write the electron configuration for neon.
Jarvas
27.10.2021 11:07Consequently the f negatron configuration will Be 1s22s22p5. Therefore the N electron configuration testament be 1s 2 2s 2 2p 3.
Onisha
28.10.2021 02:18Consequently the chlorine negatron configuration will atomic number 4 1s 2 2s 2 2p 6 3s 2 3p 5. Write the short electron configuration for c.
Yavanna
20.10.2021 10:18This is the presently selected item. Electron configurations are a right smart to show how the electrons of a particular corpuscle or ion ar present in their particle's vicinity.